Products
At Pharvis, we strive for a healthier and happier life for every family.
 The extract from horse chestnut seeds (content of aescin)
The extract from horse chestnut seeds (content of aescin)
Treatment for chronic venous insufficiency
Made and Exported by Aenova in Germany


The company headquarters are established in Munich.
The Group initially has sever production sites in five countries (Germany, Switzerland, France, Romania, and the USA) in 2012 the Temmler Group is acquired
Phavis Korea Pharma Co., Ltd. has signed an exclusive agreement with Aenova for the import and sale of Venostasin Capsule in 2018
1. Venostasin® retard is approved for the
"treatment of complaints associated with
diseases of the leg veins
(chronic venous insufficiency)
Chronic venous insufficiency (CVI) is a condition that occurs when the venous wall and/or valves in the leg veins are not working effectively, making it difficult for blood to return to the heart from the legs. CVI causes blood to “pool” or collect in these veins, and this pooling is called stasis. Symptoms include “pain and sensation of heaviness in the legs, nocturnal calf cramps, pruritus and swollen legs”
2. Evidence based Efficacy
Aescin, an active ingredient in Venostasin Cap, is very effective for chronic venous insufficiency symptoms.
- baseline
- end of treatment
-
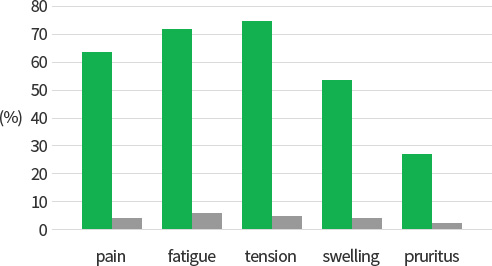
Fig 1. Reduction in the % of patients suffering from CVI with unbearable/severe symptoms after treatment with aescin for 4~10 weeks in an observational trial in over 5,000 patients in clinical practice
-

Fig 2. Increase in the % of patients suffering from CVI free of symptoms after treatment with aescin for 4~10 weeks in an ovservational trial in over 5,000 patients in clinical practice

Fig 6. Changes in lower leg volume during the study period(mean value ±SD). active ereatmentt(HCSE and Compression) versus plaoebo
- placebo
- aescin
-
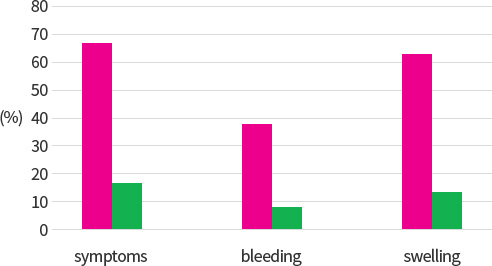
Fig 3. Persistence of acute signs and symptoms in a double-blind. placebo-controlled. parallel group trial in patieents with acute hemorrgoidal attacks after treatment with either 40mg aescin tid orally (n=38) or matching placebo(n=34)
Equivalent to compression stocking in terms of edema reduction
-
Aescin dosage group Placebo group Target Hand-related surgical patients Number of patients 27명 26명 Hand
Temperature2 days after surgery,
rapid decrease after
Peak Hand Temperature4 days after surgery,
gradually decrease after
Peak Hand TemperatureEffective in improving hemorrhoid symptoms and postoperative edema.









